Professor
NOMIS Center for Immunobiology and Microbial Pathogenesis
Becky and Ralph S. O'Connor Chair

The immune system is a powerful, double-edged sword. On one hand, it is armed to fight a wide range of invading foreign pathogens. On the other hand, if left unchecked, it can also attack an organism’s own tissues and cause inflammation and autoimmune disorders such as allergies, asthma, rheumatoid arthritis, multiple sclerosis and type 1 diabetes. There are multiple safeguards built into our cells to prevent an autoimmune reaction, but these can go haywire. What’s more, some types of cancer can also evade or co-opt the immune system’s detection, allowing tumor cells to proliferate.
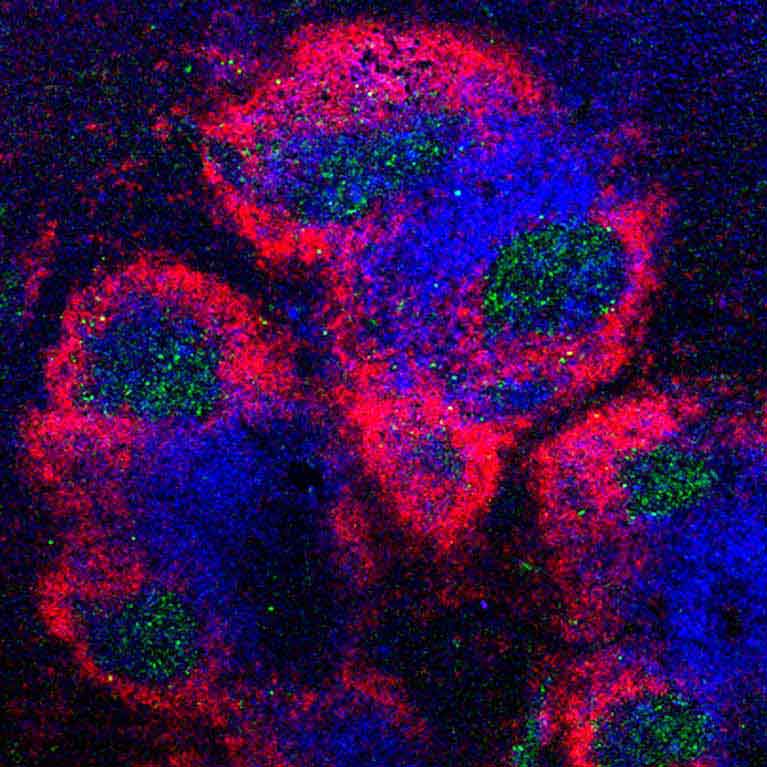
To learn how to strengthen or correct the immune system, Ye Zheng focuses on a specialized set of immune cells called regulatory T (Treg) cells. Tregs control the immune response, telling the more aggressive immune cells when to stop their frenzied attack. Abnormal Treg cell function has been linked to multiple autoimmune diseases and tumors. In particular, a key molecular component of these cells, a protein called Foxp3, is often responsible for deficient Tregs. Zheng is making advances in understanding the genes that control Foxp3—as well as genes that Foxp3 controls—to ultimately lead to ways to manage Treg cell function. Since manipulations of Treg cells can either weaken or strengthen the immune response, his findings can potentially open new avenues in the treatment of autoimmune diseases, improve organ transplant survival and uncover new cancer targets.
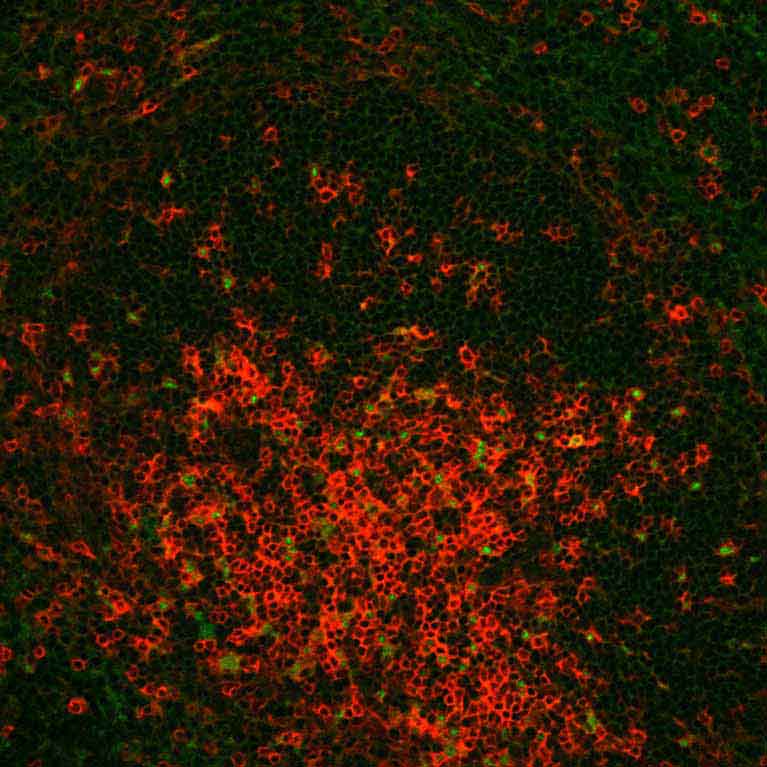
Zheng has mapped hundreds of genes directly related to Tregs’ Foxp3 protein to get a fuller picture of how these cellular peacekeepers develop and function.
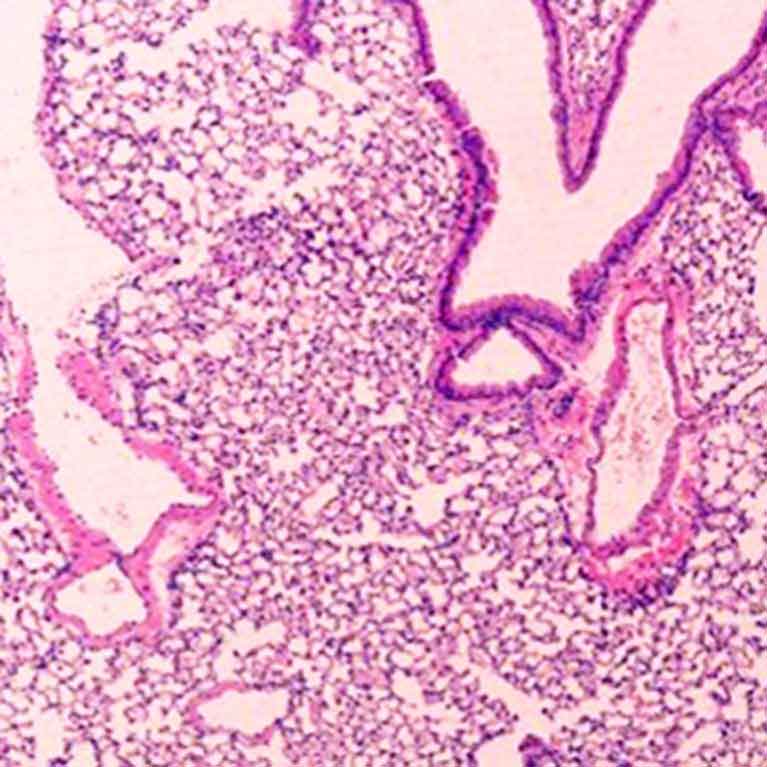
Zheng’s lab discovered that a particular genetic sequence in Foxp3 (called CNS2) is responsible for the stability of a Treg cell. If the team removed CNS2, Treg cells became unstable and often morphed into killer T cells—the type of cell Tregs are supposed to be controlling—resulting in autoimmune disease in animals.
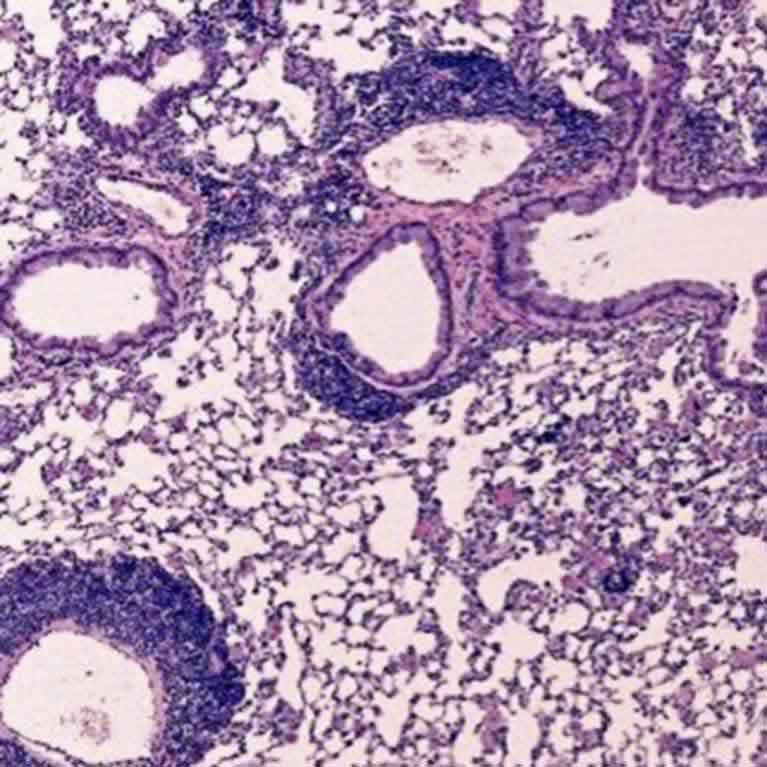
His team identified a group of proteins directly regulated by Foxp3 that drives Treg cell function. These proteins can be targeted to boost Treg cell function for treatment of autoimmune diseases such as type 1 diabetes, allergy and asthma.
BS, Biochemistry and Molecular Biology, Peking University, Beijing, China
PhD, Columbia University
Postdoctoral Fellow, University of Washington
Memorial Sloan-Kettering Cancer Center Research Scholar
Cancer Research Institute Postdoctoral Fellowship