
August 25, 2016
Salk Institute tests drug that could boost levels of critical protective protein in brain
Salk Institute tests drug that could boost levels of critical protective protein in brain
LA JOLLA—Boosting levels of a specific protein in the brain alleviates hallmark features of Alzheimer’s disease in a mouse model of the disorder, according to new research published online August 25, 2016 in Scientific Reports.
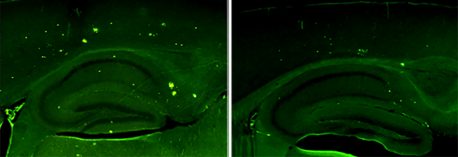
Click here for a high-resolution image.
Credit: Salk Institute
The protein, called neuregulin-1, has many forms and functions across the brain and is already a potential target for brain disorders such as Parkinson’s disease, amyotrophic lateral sclerosis and schizophrenia.
“Neuregulin-1 has broad therapeutic potential, but mechanistically, we are still learning about how it works,” says the study’s senior investigator Kuo-Fen Lee, a professor in the Salk Institute’s Clayton Foundation Laboratories for Peptide Biology and holder of the Helen McLoraine Chair in Molecular Neurobiology. “We’ve shown that it promotes metabolism of the brain plaques that are characteristic of Alzheimer’s disease.”
Previously, researchers have shown that treating cells with neuregulin-1, for example, dampens levels of amyloid precursor protein, a molecule that generates amyloid beta, which aggregate and form plaques in the brains of Alzheimer’s patients. Other studies suggest that neuregulin-1 could protect neurons from damage caused by blockage of blood flow.
In the new study, Lee’s team tested this idea in a mouse model of Alzheimer’s disease by raising the levels of one of two forms of neuregulin-1 in the hippocampus, an area of the brain responsible for learning and memory. Both forms of the protein seemed to improve performance on a test of spatial memory in the models.
What’s more, the levels of cellular markers of disease—including the levels of amyloid beta and plaques—were noticeably lower in mice with more neuregulin-1 compared to controls.
Click here for a high-resolution image
Credit: Salk Institute
The group’s experiments suggest that neuregulin-1 breaks up plaques by raising levels of an enzyme called neprilysin, shown to degrade amyloid-beta. But that is probably not the only route through which neuregulin-1 confers its benefits, and the group is exploring other possible mechanisms—such as whether the protein improves signaling between neurons, which is impaired in Alzheimer’s—says the study’s first author Jiqing Xu, a research associate in Lee’s group.
A neuregulin-1 treatment is not available on the market, though it is being explored in clinical trials as a potential treatment for chronic heart failure and Parkinson’s disease. One advantage of neuregulin-1 as a potential drug is that it can cross the blood brain barrier, which means that it could be administered relatively noninvasively even though the efficiency is not clear. On the other hand, other research suggests too much of the protein impairs brain function. Working with chemists at Salk, Lee’s team has come up with a small molecule that can raise levels of existing neuregulin-1 (rather than administering it directly) and are testing it in cells. This alternative therapy could be a better way to prevent plaques from forming because small molecules more readily cross the blood brain barrier.
The group is also interested in neuregulin-1 for its ties to schizophrenia. An alteration in the neuregulin-1 gene—a single change in one letter of the DNA code for the protein—has been found in families with schizophrenia and linked to late-onset Alzheimer’s disease with psychosis. The protein may be a way to understand the overlap between Alzheimer’s and other brain disorders, Lee says.
An important caveat is that the new research was conducted in a single type of mouse model of Alzheimer’s. Lee’s group is testing neuregulin-1’s affects across other models. “There’s much more work ahead before neuregulin-1 could become a treatment, but we are excited about its potential, possibly in combination with other therapeutics for Alzheimer’s disease,” Lee says.
Other authors on the study are Fred DeWinter, Catherine Farrokhi, Jonathan Cook and Xin Jin of Salk’s Clayton Foundation for Peptide Biology Laboratories; and Edward Rockenstein, Michael Mante, Anthony Adame and Eliezer Masliah of the University of California, San Diego.
The research was supported by the National Institutes of Health, the Clayton Foundation, The Albert G. and Olive H. Schlink Foundation, the Gemcon Family Foundation and the Brown Foundation.
JOURNAL
Scientific Reports
AUTHORS
Jiqing Xu, Fred DeWinter, Catherine Farrokhi, Edward Rockenstein, Michael Mante, Anthony Adame, Jonathan Cook, Xin Jin, Eliezer Masliah and Kuo-Fen Lee
Office of Communications
Tel: (858) 453-4100
press@salk.edu
Unlocking the secrets of life itself is the driving force behind the Salk Institute. Our team of world-class, award-winning scientists pushes the boundaries of knowledge in areas such as neuroscience, cancer research, aging, immunobiology, plant biology, computational biology and more. Founded by Jonas Salk, developer of the first safe and effective polio vaccine, the Institute is an independent, nonprofit research organization and architectural landmark: small by choice, intimate by nature, and fearless in the face of any challenge.