
July 30, 2019
Salk scientists uncover a mechanism linking a protein mutation with abnormal nervous system development
Salk scientists uncover a mechanism linking a protein mutation with abnormal nervous system development
LA JOLLA—Neurodevelopmental disorders arising from rare genetic mutations can cause atypical cognitive function, intellectual disability, and developmental delays, yet it is unclear why and how this happens. Scientists suspected a mutation in a complex of proteins could be the culprit for a group of rare genetic disorders and, now, Salk Institute researchers have identified the molecular mechanism linking this mutation with abnormal nervous system development. The team’s findings, published in Molecular Cell on July 30, 2019, bring researchers one step closer to understanding neurodevelopmental disorders, such as Nicolaides-Baraitser syndrome and others.
“For the first time, we have been able to characterize the mechanism of a known gene mutation implicated in neurodevelopmental disorders,” says Assistant Professor Diana Hargreaves, senior author and holder of the Richard Heyman and Anne Daigle Endowed Developmental Chair.
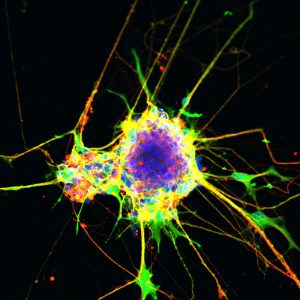
Click here for a high-resolution image.
Credit: Salk Institute
The root cause has to do with a complex of proteins called the SWI/SNF complex, which is involved in DNA regulation, and, when mutated, is associated with Nicolaides-Baraitser syndrome, Coffin-Siris syndrome, autism and even some cancers. These complexes repackage and reshape the DNA in the nucleus to either enable or prevent access to genes. And yet, scientists do not know how mutations in individual subunits of the SWI/SNF complex affect its function.
“We sought to understand how a single mutation in the SMARCA2 subunit affected brain development,” says Fangjian Gao, the paper’s first author and a postdoctoral fellow at Salk. “We expected to see some effect on the neurodevelopmental pathways, but we were not sure how, specifically.”

Click here for a high-resolution image.
Credit: Salk Institute
The scientists turned to cell cultures in a dish to model growth patterns in afflicted brain cells versus normal brain cells. They used the gene-editing technique CRISPR to mimic the SMARCA2 mutation observed in Nicolaides-Baraitser syndrome. Notably, the researchers found that healthy cells had minimal SMARCA2 activity. The cells with the mutation, however, had a dramatic increase in SMARCA2 activity and a significantly impaired ability to generate precursors to neurons, called neural progenitor cells. In this highly activated state, SMARCA2 affected the function of the normal SWI/SNF complex. This led to a domino effect with changes in gene expression resulting in abnormal brain development.
“By better understanding this mutation in SMARCA2, we have tapped into what looks like a core developmental process that could be perturbed in disease states such as autism or even cancer,” says Hargreaves.
Other authors included Nicholas J. Elliott, Josephine Ho and Maxim N. Shokhirev of Salk, along with Alexzander Sharp of the University of California San Diego.
The work was funded by the Helmsley Trust, the National Institutes of Health (9R35 GM128943-01 and R00 CA184043-03) and the V Foundation for Cancer Research (V2016-006).
DOI: 10.1016/j.molcel.2019.06.024
JOURNAL
Molecular Cell
TITLE
Heterozygous mutations in SMARCA2 reprogram the enhancer landscape by global retargeting of SMARCA4
AUTHORS
Fangjian Gao, Nicholas J. Elliott, Josephine Ho, Alexzander Sharp, Maxim N. Shokhirev and Diana C. Hargreaves
JOURNAL COVER

Office of Communications
Tel: (858) 453-4100
press@salk.edu
Unlocking the secrets of life itself is the driving force behind the Salk Institute. Our team of world-class, award-winning scientists pushes the boundaries of knowledge in areas such as neuroscience, cancer research, aging, immunobiology, plant biology, computational biology and more. Founded by Jonas Salk, developer of the first safe and effective polio vaccine, the Institute is an independent, nonprofit research organization and architectural landmark: small by choice, intimate by nature, and fearless in the face of any challenge.