
August 16, 2022
Findings could lead to treatments for fear-related disorders
Findings could lead to treatments for fear-related disorders
LA JOLLA—Salk scientists have uncovered a molecular pathway that distills threatening sights, sounds and smells into a single message: Be afraid. A molecule called CGRP enables neurons in two separate areas of the brain to bundle threatening sensory cues into a unified signal, tag it as negative and convey it to the amygdala, which translates the signal into fear.
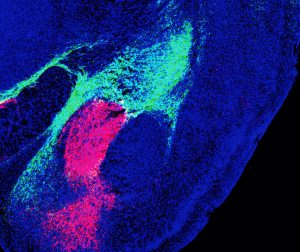
The research, published in Cell Reports on August 16, 2022, may lead to new therapies for fear-related disorders such as post-traumatic stress disorder (PTSD) or hypersensitivity disorders such as autism, migraines and fibromyalgia.
“The brain pathway we discovered works like a central alarm system,” says senior author Sung Han, assistant professor in Salk’s Clayton Foundation Laboratories for Peptide Biology. “We were excited to find that the CGRP neurons are activated by negative sensory cues from all five senses—sight, sound, taste, smell and touch. Identifying new threat pathways provides insights into treating fear-related disorders.”
Most external threats involve multisensory cues, such as the heat, smoke and smell of a wildfire. Previous research showed that different pathways independently relay sound, sight, and touch threat cues to multiple brain areas. A single pathway that integrates all these cues would be beneficial to survival, but no one had ever found such a pathway.
Previous research also showed that the amygdala, which initiates behavioral responses and forms fear memories to environmental and emotional stimuli, receives heavy input from brain regions that are laden with a chemical associated with aversion, the neuropeptide CGRP (calcitonin gene-related peptide).

“Based on these two pools of research, we proposed that CGRP neurons, found especially in subregions of the thalamus and the brainstem, relay multisensory threat information to the amygdala,” says co-first author Shijia Liu, a graduate student in the Han lab. “These circuits may both generate appropriate behavioral responses and help form aversive memories of threat cues.”
The team conducted several experiments to test their hypotheses. They recorded CGRP neuron activity using single-cell calcium imaging while presenting mice with multisensory threat cues, enabling the researchers to pinpoint which sensory modality involved which sets of neurons. They determined the path the signals took after leaving the thalamus and brainstem using different colored fluorescent proteins. And they conducted behavioral tests to gauge memory and fear.
Taken together, their findings show that two distinct populations of CGRP neurons—one in the thalamus, one in the brainstem—project to nonoverlapping areas of the amygdala, forming two distinct circuits. Both populations encode threatening sights, sounds, smells, tastes and touches by communicating with local brain networks. Finally, they discovered that both circuits are necessary for forming aversive memories—the kind that tell you, “Stay away.”

“While mice were used in this study, the same brain regions also abundantly express CGRP in humans,” says Han, holder of the Pioneer Fund Developmental Chair. “This suggests that the circuits reported here may also be involved in threat perception-related psychiatric disorders.”
The authors hope to examine how CGRP signaling in these circuits mediates disorders involving multisensory stimuli processing abnormalities, such as migraines, PTSD and autism spectrum disorder.
“We haven’t tested it yet, but migraines might also activate these CGRP neurons in the thalamus and brainstem,” says co-first author Sukjae Joshua Kang, a postdoctoral fellow in the Han lab. “Drugs that block CGRP have been used to treat migraines, so I’m hoping that our study can be an anchor to use this kind of drug in relieving threat memories in PTSD, or sensory hypersensitivity in autism, too.”
Other authors included Mao Ye, Dong-Il Kim, Gerald M. Pao and Kuo-Fen Lee of Salk; Bryan A. Copits of Washington University in St. Louis; Benjamin Z. Roberts of UC San Diego; and Michael R. Bruchas of University of Washington.
The work was supported in part by the National Institute of Mental Health (1R01MH116203; 1R01MH111520; R01MH112355), Simons Foundation Autism Research Initiative (Bridge to Independence award SFARI #388708), Salk Women & Science Special Award, Mary K. Chapman Foundation, and Jesse & Caryl Philips Foundation.
DOI: 10.1016/j.celrep.2022.111222
JOURNAL
Cell Reports
AUTHORS
Sukjae Joshua Kang, Shijia Liu, Mao Ye, Dong-Il Kim, Gerald M. Pao, Bryan A. Copits, Benjamin Z. Roberts, Kuo-Fen Lee, Michael R. Bruchas, Sung Han
Office of Communications
Tel: (858) 453-4100
press@salk.edu
Unlocking the secrets of life itself is the driving force behind the Salk Institute. Our team of world-class, award-winning scientists pushes the boundaries of knowledge in areas such as neuroscience, cancer research, aging, immunobiology, plant biology, computational biology and more. Founded by Jonas Salk, developer of the first safe and effective polio vaccine, the Institute is an independent, nonprofit research organization and architectural landmark: small by choice, intimate by nature, and fearless in the face of any challenge.