
November 11, 2010
LA JOLLA, CA—A collaborative effort between researchers at the Salk Institute for Biological Studies and the University of California, San Diego, successfully used human induced pluripotent stem (iPS) cells derived from patients with Rett syndrome to replicate autism in the lab and study the molecular pathogenesis of the disease.
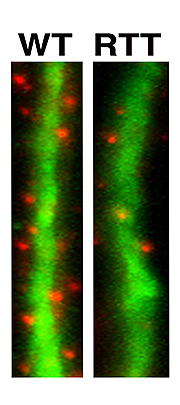
Neurons generated from Rett-iPS cells form fewer synapses, the specialized signal transmission points between brain cells. Synapses are shown in red and dendrites, which function as signal receivers, are shown in green.
Image: Courtesy of Dr. Carol Marchetto, Salk Institute for Biological Studies.
Their findings, published in the Nov. 12, 2010, issue of Cell, revealed disease-specific cellular defects, such as fewer functional connections between Rett neurons, and demonstrated that these symptoms are reversible, raising the hope that, one day, autism maybe turn into a treatable condition.
“Mental disease and particularly autism still carry the stigma of bad parenting,” says lead author Alysson Muotri, Ph.D., an assistant professor in the Department of Molecular and Cellular Medicine at the University of California, San Diego School of Medicine. “We show very clearly that autism is a biological disease that is caused by a developmental defect directly affecting brain cells.”
Rett syndrome is the most physically disabling of the autism spectrum disorders. Primarily affecting girls, the symptoms of Rett syndrome often become apparent just after they have learned to walk and say a few words. Then, the seemingly normal development slows down and eventually the infants regress, loosing speech and motor skills, developing stereotypical movements and autistic characteristics.
Almost all cases of the disease are caused by a single mutation in the MeCP2 gene, which is involved in the regulation of global gene expression, leading to a host of symptoms that can vary widely in their severity.
“Rett syndrome is sometimes considered a ‘Rosetta Stone’ that can help us to understand other developmental neurological disorders since it shares genetic links with other conditions such as autism and schizophrenia,” says first author Carol Marchetto, Ph.D., a postdoctoral researcher in the Laboratory of Genetics at the Salk Institute.
In the past, scientists had been limited to study the brains of people with autistic spectrum disorders via imaging technologies or postmortem brain tissues. Now, the ability to obtain iPS cells from patients’ skin cells, which can be encouraged to develop into the cell type damaged by the disease gives scientists an unprecedented view of autism.
“It is quite amazing that we can recapitulate a psychiatric disease in a Petri Dish,” says lead author Fred Gage, Ph.D., a professor in the Salk’s Laboratory of Genetics and holder of the Vi and John Adler Chair for Research on Age-Related Neurodegenerative Diseases. “Being able to study Rett neurons in a dish allows us to identify subtle alterations in the functionality of the neuronal circuitry that we never had access to before.”
Marchetto started with skin biopsies taken from four patients carrying four different mutations in the MeCP2 gene and a healthy control. By exposing the skin cells to four reprogramming factors, she turned back the clock, triggering the cells to look and act like embryonic stem cells. Known at this point as induced pluripotent stem cells, the Rett-derived cells were indistinguishable from their normal counterparts.
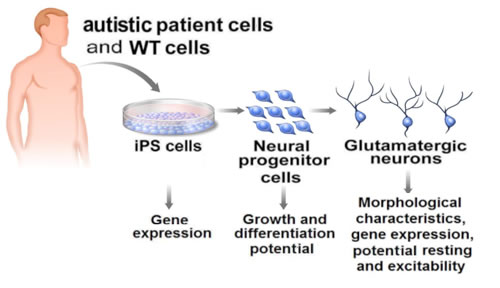
Human induced pluripotent stem (iPS) cells derived from patients with Rett syndrome allow researchers to replicate autism in the lab and study the molecular pathogenesis of the disease.
Illustration: Courtesy of Jamie Simon, Salk Institute for Biological Studies
It was only after she had patiently coaxed the iPS cells to develop into fully functioning neurons-a process that can take up to several months-that she was able to discern differences between the two. Neurons carrying the MeCP2 mutations had smaller cell bodies, a reduced number of synapses and dendritic spines, specialized structures that enable cell-cell communication, as well as electrophysical defects, indicating that things start to go wrong early in development.
Since insulin-like growth factor 1 (IGF-1)-a hormone which, among other things, has a role in regulating cell growth and neuronal development-was able to reverse some of the symptoms of Rett syndrome in a mouse model of disease, the Salk researchers tested whether IGF-1 could restore proper function to human Rett neurons grown in culture.
“IGF-1 treatment increased the number of synapses and spines reverting the neuronal phenotype closer to normal,” says Gage. “This suggests that the autistic phenotype is not permanent and could be, at least partially, reversible.”
Muotri is particularly excited about the prospect of finding a drug treatment for Rett syndrome and other forms of autism: “We now know that we can use disease-specific iPS cells to recreate mental disorders and start looking for new drugs based on measureable molecular defects.”
Researchers who also contributed to the work include Cassiano Carromeu and Allan Acab in the Department of Pediatrics/Cellular & Molecular Medicine at the University of California, San Diego, Diana Yu and Yangling Mu in the Laboratory of Genetics at the Salk Institute for Biological Studies, Gene Yeo in the School of Medicine at the University of California, San Diego, as well as Gong Chen in the Department of Biology at the Pennsylvania State University.
This work was supported by the Emerald Foundation Young Investigator Award, the National Institutes of Health through the NIH Director’s New Innovator Award Program, the California Institute for Regenerative Medicine, The Lookout Fund and the Picower Foundation.
About the Salk Institute for Biological Studies:
The Salk Institute for Biological Studies is one of the world’s preeminent basic research institutions, where internationally renowned faculty probe fundamental life science questions in a unique, collaborative, and creative environment. Focused both on discovery and on mentoring future generations of researchers, Salk scientists make groundbreaking contributions to our understanding of cancer, aging, Alzheimer’s, diabetes and infectious diseases by studying neuroscience, genetics, cell and plant biology, and related disciplines.
Faculty achievements have been recognized with numerous honors, including Nobel Prizes and memberships in the National Academy of Sciences. Founded in 1960 by polio vaccine pioneer Jonas Salk, M.D., the Institute is an independent nonprofit organization and architectural landmark.
The Salk Institute proudly celebrates five decades of scientific excellence in basic research.
Office of Communications
Tel: (858) 453-4100
press@salk.edu