
January 5, 2021
Salk researchers find decreased activation of gene called LEF1 is more common in neurons of people with bipolar disorder who do not respond to lithium, pointing way to potential new treatment
Salk researchers find decreased activation of gene called LEF1 is more common in neurons of people with bipolar disorder who do not respond to lithium, pointing way to potential new treatment
LA JOLLA—Lithium is considered the gold standard for treating bipolar disorder (BD), but nearly 70 percent of people with BD don’t respond to it. This leaves them at risk for debilitating, potentially life-threatening mood swings. Researchers at the Salk Institute have found that the culprit may lie in gene activity—or lack of it.
A new study led by Salk Professor and President Rusty Gage, which published in the journal Molecular Psychiatry on January 4, 2021, shows that decreased activation of a gene called LEF1 disrupts ordinary neuronal function and promotes hyperexcitability in brain cells—a hallmark of BD. The work could result in a new drug target for BD as well as a biomarker for lithium nonresponsiveness.
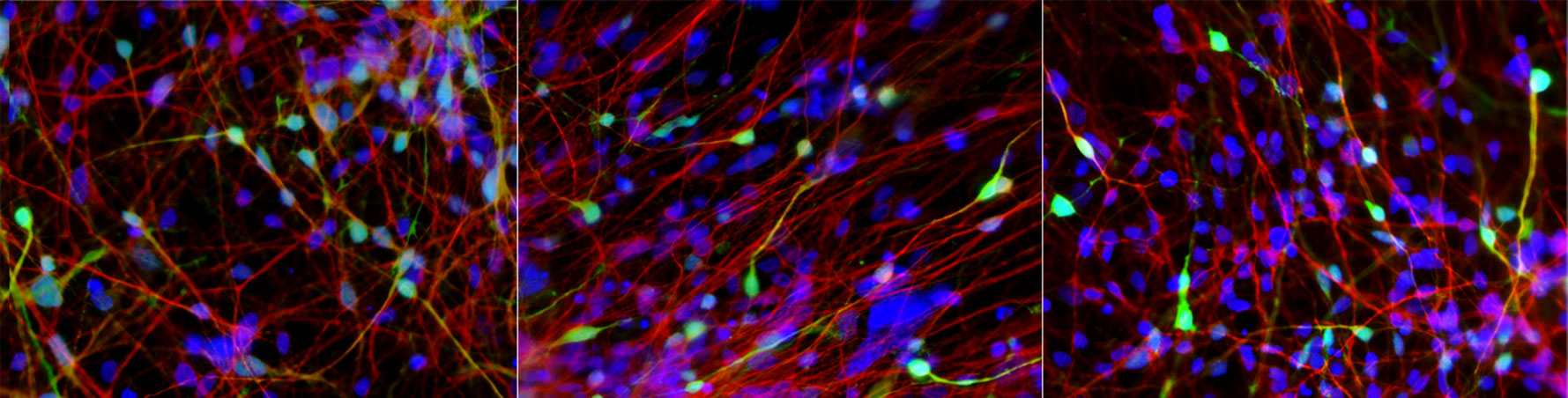
Click here for a high-resolution image.
Credit: Salk Institute
“Only one-third of patients respond to lithium with disappearance of the symptoms,” says Renata Santos, co-first author on the study and a Salk research collaborator. “We were interested in the molecular mechanisms behind lithium resistance, what was blocking lithium treatment in nonresponders. We found that LEF1 was deficient in neurons derived from nonresponders. We were excited to see that it was possible to increase LEF1 and its dependent genes, making it a new target for therapeutic intervention in BD.”
The study builds on the team’s earlier findings, which reported that the neurons of people with BD who don’t respond to lithium are larger, fire differently (are more easily stimulated, or hyperexcitable), and have increased potassium flow.
Subjects in the team’s current study included lithium responders, nonresponders and people without BD (controls). Using stem-cell methods, the researchers grew neurons from the subjects’ blood cells and compared the genetic disposition and behavior of the neurons for the three groups.
They looked at many genes across the board, but LEF1 stood out as one of the most different in nonresponders. Normally, LEF1 plays a decisive role in neuronal function by pairing with another protein called beta-catenin. The pairing typically activates other genes that regulate the level of activity in the neuron. In control or responding neurons, lithium enables beta-catenin to pair with LEF1. But in nonresponders, lithium is ineffective because LEF1 levels are too low for the pairing to occur, so there’s no regulation of cell activity.
When the team administered valproic acid, a treatment often used for nonresponders, measurements showed increased levels of LEF1 and activation of the other relevant genes. And when the team silenced the LEF1 gene in control neurons, they found that the related genes were not activated. Together, these results indicate the critical role LEF1 plays in controlling neuronal hyperexcitability.
“When we silenced the LEF1 gene, the neurons became hyperexcitable,” says Shani Stern, co-first author on the study and a Salk visiting scientist. “And when we used valproic acid, expression of LEF1 increased, and we lowered the hyperexcitability. That shows there is a causative relationship, and that’s why we think LEF1 may be a possible target for drug therapy.”
LEF1 may also help researchers develop a screening test for responsiveness. Currently, clinicians can only determine whether a patient is responsive to lithium by administering a complete course of treatment, which could take a year. Now, subdued activity of LEF1 may be an indicator that a patient won’t respond to lithium, enabling a faster and more efficient way to approach therapy.
Team members already contemplating next steps. These include looking at other cell types, such as astrocytes and GABAergic neurons, to understand the bipolar neural network as a whole; identifying other genes that could be beneficial for nonresponders; and finding other drugs that can activate LEF1.
“LEF1 works in various ways in different parts of the body, so you can’t just turn it on everywhere,” says Carol Marchetto, co-corresponding author and Salk research collaborator. “You want to be more specific, either activating LEF1 on a targeted basis or activating downstream genes that are relevant for lithium nonresponsiveness.”
Other authors on the study were Sara B. Linker, Ana P. D. Mendes, Lynne Randolph-Moore, Vipula Racha, Yeni Kim, Maxim N. Shokhirev, and Galina Erikson of Salk; John R. Kelsoe of the University of California San Diego; Anne G. Bang of the Sanford Burnham Prebys Medical Discovery Institute; and M. Alda of Dalhousie University.
The work was funded by the National Institutes of Health, the Chapman Foundation and the Helmsley Charitable Trust, the National Cancer Institute, the National Cooperative Reprogrammed Cell Research Groups, the JPB Foundation, Annette C. Merle-Smith, the Robert and Mary Jane Engman Foundation, and the Zuckerman STEM leadership program funding.
DOI: 10.1038/s41380-020-00981-3
JOURNAL
Molecular Psychiatry
AUTHORS
Renata Santos, Sara B. Linker, Shani Stern, Ana P. D. Mendes, Maxim N. Shokhirev, Galina Erikson, Lynne Randolph-Moore, Vipula Racha, Yeni Kim, John R. Kelsoe, Anne G. Bang, M. Alda, Maria C. Marchetto, Fred H. Gage
Office of Communications
Tel: (858) 453-4100
press@salk.edu
Unlocking the secrets of life itself is the driving force behind the Salk Institute. Our team of world-class, award-winning scientists pushes the boundaries of knowledge in areas such as neuroscience, cancer research, aging, immunobiology, plant biology, computational biology and more. Founded by Jonas Salk, developer of the first safe and effective polio vaccine, the Institute is an independent, nonprofit research organization and architectural landmark: small by choice, intimate by nature, and fearless in the face of any challenge.