
April 6, 2017
The new technique, which allows scientists to generate both embryonic and non-embryonic tissues from cultured stem cells, is a step toward growing donor organs and replacement tissues to combat aging and diseases
The new technique, which allows scientists to generate both embryonic and non-embryonic tissues from cultured stem cells, is a step toward growing donor organs and replacement tissues to combat aging and diseases
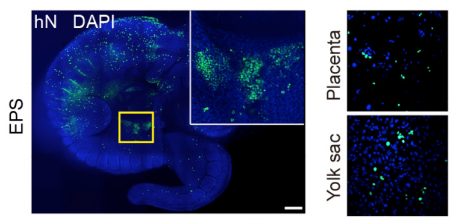
LA JOLLA—When scientists talk about laboratory stem cells being totipotent or pluripotent, they mean that the cells have the potential, like an embryo, to develop into any type of tissue in the body. What totipotent stem cells can do that pluripotent ones can’t do, however, is develop into tissues that support the embryo, like the placenta. These are called extra-embryonic tissues, and are vital in development and healthy growth.
Click here for a high-resolution image
Credit: Salk Institute
Now, scientists at the Salk Institute, in collaboration with researchers from Peking University, in China, are reporting their discovery of a chemical cocktail that enables cultured mouse and human stem cells to do just that: generate both embryonic and extra-embryonic tissues. Their technique, described in the journal Cell on April 6, 2017, could yield new insights into mammalian development that lead to better disease modeling, drug discovery and even tissue regeneration. This new technique is expected to be particularly useful for modeling early developmental processes and diseases affecting embryo implantation and placental function, possibly paving the way for improved in vitro fertilization techniques.
“During embryonic development, both the fertilized egg and its initial cells are considered totipotent, as they can give rise to all embryonic and extra-embryonic lineages. However, the capture of stem cells with such developmental potential in vitro has been a major challenge in stem cell biology,” says Salk Professor Juan Carlos Izpisua Bemonte, co–senior author of the paper and holder of Salk’s Roger Guillemin Chair. “This is the first study reporting the derivation of a stable stem cell type that shows totipotent-like bi-developmental potential towards both embryonic and extra-embryonic lineages.”
Once a mammalian egg is fertilized and begins dividing, the new cells segregate into two groups: those that will develop into the embryo and those that will develop into supportive tissues like the placenta and amniotic sac. Because this division of labor happens relatively early, researchers often can’t maintain cultured cell lines stably until cells have already passed the point where they could still become either type. The newly discovered cocktail gives stem cells the ability to stably become either type, leading the Salk team to dub them extended pluripotent stem (EPS) cells.

Click here for a high-resolution image
Credit: Salk Institute
“The discovery of EPS cells provides a potential opportunity for developing a universal method to establish stem cells that have extended developmental potency in mammals,” says Jun Wu, a senior scientist at Salk and one of the paper’s first authors. “Importantly, the superior interspecies chimeric competency of EPS cells makes them especially valuable for studying development, evolution and human organ generation using a host animal species.”
To develop their cocktail, the Salk team, together with the team from Peking University, first screened for chemical compounds that support pluripotency. They discovered that a simple combination of four chemicals and a growth factor could stabilize the human pluripotent stem cells at a developmentally less mature state, thereby allowing them to more efficiently contribute to chimera (a mix of cells from two different species) formation in a developing mouse embryo. They also applied the same factors to mouse cells and found, surprisingly, that the newly derived mouse stem cells could not only give rise to embryonic tissue types but also differentiate into cells from the extra-embryonic lineages. Moreover, the team found that the new mouse stem cells have a superior ability to form chimeras and a single cell could give rise to an entire adult mouse, which is unprecedented in the field, according to the team.
“The superior chimeric competency of both human and mouse EPS cells is advantageous in applications such as the generation of transgenic animal models and the production of replacement organs,” adds Wu. “We are now testing to see whether human EPS cells are more efficient in chimeric contribution to pigs, whose organ size and physiology are closer to humans.” Human EPS cells, combined with the interspecies blastocyst complementation platform as reported by the same Salk team in Cell in January 2017, hold great potential for the generation of human organs in pigs to meet the rising demand for donor organs.
“We believe that the derivation of a stable stem cell line with totipotent-like features will have a broad and resounding impact on the stem cell field,” says Izpisua Belmonte.
Other authors included: Takayoshi Yamauchi, Atsushi Sugawara and Zhongwei Li of Salk; Yang Yang, Bei Liu, Jun Xu, Jinlin Wang, Cheng Shi, Yaxing Xu, Jiebin Dong, Chengyan Wang, Weifeng Lai, Jialiang Zhu, Liang Xiong, Dicong Zhu, Xiang Li, Chen Li, Aibin He, Yaqin Du, Ting Wang, Chaoran Zhao, Haibo Li, Hongquan Zhang, Xiaochun Chi, and Huan Shen of Peking University; Weifeng Yang and Ming Yin of Beijing Vitalstar Biotechnology; Fangyuan Sun and Xiangyun Li of Hebei University; Yifang Liu of Tsinghua University; Cheng Li of Peking-Tsinghua Center for Life Sciences; Shuguang Duo of the Chinese Academy of Sciences.
The work was funded by: the National Key Research and Development Program of China (2016YFA0100100), the National Natural Science Foundation of China (31521004), the Guangdong Innovative and Entrepreneurial Research Team Program (2014ZT05S216), the Science and Technology Planning Project of Guangdong Province, China (2014B020226001), the Science and Technology Program of Guangzhou, China (2016B030232001), the Ministry of Education of China (111 Project), the BeiHao Stem Cell and Q9 Regenerative Medicine Translational Research Institute, the Joint Institute of Peking University Health Science Center, University of Michigan Health System, Peking-Tsinghua Center for Life Sciences, the National Science and Technology Support Project (2014BAI02B01), the CAS Key Technology Talent Program, the G. Harold and Leila Y. Mathers Charitable Foundation, and The Moxie Foundation.
JOURNAL
Cell
AUTHORS
Yang Yang, Bei Liu, Jun Xu, Jinlin Wang, Jun Wu, Cheng Shi, Yaxing Xu, Jiebin Dong, Chengyan Wang, Weifeng Lai, Jialiang Zhu, Liang Xiong, Dicong Zhu, Xiang Li, Weifeng Yang, Takayoshi Yamauchi, Atsushi Sugawara, Zhongwei Li, Fangyuan Sun, Xiangyun Li, Chen Li, Aibin He, Yaqin Du, Ting Wang, Chaoran Zhao, Haibo Li, Xiaochun Chi, Hongquan Zhang, Yifang Liu, Cheng Li, Shuguang Duo, Ming Yin, Huan Shen, Juan Carlos Izpisua Belmonte, and Hongkui Deng
Office of Communications
Tel: (858) 453-4100
press@salk.edu
Unlocking the secrets of life itself is the driving force behind the Salk Institute. Our team of world-class, award-winning scientists pushes the boundaries of knowledge in areas such as neuroscience, cancer research, aging, immunobiology, plant biology, computational biology and more. Founded by Jonas Salk, developer of the first safe and effective polio vaccine, the Institute is an independent, nonprofit research organization and architectural landmark: small by choice, intimate by nature, and fearless in the face of any challenge.