
May 1, 2018
The Salk approach, tested in a mouse model, points to cheaper and more effective treatments for humans
The Salk approach, tested in a mouse model, points to cheaper and more effective treatments for humans
LA JOLLA—For most people with hemophilia B, whose bodies can’t properly form blood clots, constant injections to replenish their clotting factors are a way of life. But now, Salk researchers have demonstrated in mice that hemophilia B can be treated for life with one single injection containing disease-free liver cells that can produce their missing clotting factor. The finding, published in the journal Cell Reports on May 1, 2018, could drastically change what it means to be diagnosed with hemophilia B, and could pave the way toward similar treatments for other, related genetic disorders.
Click here for a high-resolution image.
Credit: Salk Institute
Hemophilia B is caused by defects in the gene for a protein called clotting factor IX (FIX). Hemophiliacs may make reduced amounts of the protein, or lack a functional version altogether, leading to life-threatening delays in blood clotting. Currently, patients are treated with injections—as often as a few times a week—containing FIX made in animal cells and then purified. But the approach is expensive, time-consuming and can become less effective over time.
Recently, Salk scientists developed a new approach, treating mice genetically engineered to have hemophilia B with strands of messenger RNA encoding the FIX gene. Like the standard treatment, however, this required repeat injections each time levels of the messenger RNA ran low. So the scientists wanted to try a more permanent approach: transplanting healthy liver cells, capable of producing FIX, into patients.
“The appeal of a cell-based approach is that you minimize the number of treatments that a patient needs,” says Suvasini Ramaswamy, a former Salk research associate and first author of the new paper. “Rather than constant injections, you can do this in one shot.”
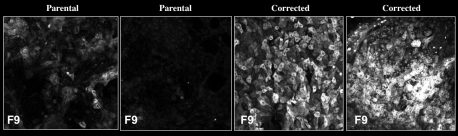
Since donor livers are often in short-supply, the researchers instead turned to stem-cell strategies to produce the healthy liver cells. They collected blood samples from two human patients with severe hemophilia B, who are unable to produce FIX. Then, in the lab, they reprogrammed the cells into induced pluripotent stem cells (iPSCs), which have the capability to turn into many other cell types, including liver. Using CRISPR/Cas9, a tool that can alter genes, they then repaired the mutations in each patient’s FIX gene. Finally, they coaxed those repaired cells to develop into liver precursor cells called hepatocyte-like cells (HLCs) and transplanted them into mice with hemophilia B.
Rather than perform surgery on hemophilic mice—a risky undertaking when their blood can’t always clot—the team transplanted the HLCs through the spleen to distribute the cells uniformly in the liver.
Not only did the new HLCs produce FIX, but they produced enough of the protein to allow the mice to form normal blood clots, and the cells continued to survive—and produce FIX—for at least a year after the transplantation.
In people with hemophilia, using their own cells to generate healthy HLCs, then transplanting them back into their bodies, could help avoid the immune complications that often accompany cell therapies. But more work is needed to translate the findings to the clinic.
“A lot of things have to happen before this can go into humans,” says Ramaswamy.
Already, she adds, the work demonstrates the value in combining stem-cell reprogramming and new gene-modifying approaches to treat genetic diseases.
The work and the researchers involved were supported by grants from the National Institutes of Health, the National Cancer Institute, the National Institute of Neurological Disorders and Stroke, the Waitt Foundation, Ipsen, the H. N. and Frances C. Berger Foundation, the Glenn Center for Aging Research, the Leona M. and Harry B. Helmsley Charitable Trust, and the California Institute for Regenerative Medicine.
JOURNAL
Cell Reports
TITLE
Autologous and heterologous cell therapy for hemophilia B towards functional restoration of Factor IX
AUTHORS
Suvasini Ramaswamy, Nina Tonnu, Tushar Menon, Benjamin M. Lewis, Kevin T. Green, Derek Wampler, Paul E. Monahan and Inder M. Verma
Office of Communications
Tel: (858) 453-4100
press@salk.edu
Unlocking the secrets of life itself is the driving force behind the Salk Institute. Our team of world-class, award-winning scientists pushes the boundaries of knowledge in areas such as neuroscience, cancer research, aging, immunobiology, plant biology, computational biology and more. Founded by Jonas Salk, developer of the first safe and effective polio vaccine, the Institute is an independent, nonprofit research organization and architectural landmark: small by choice, intimate by nature, and fearless in the face of any challenge.