
October 22, 2024
Salk scientists achieve cross-chiral exponential amplification, potentially enabling a new form of artificial life and innovative drug development strategies
Salk scientists achieve cross-chiral exponential amplification, potentially enabling a new form of artificial life and innovative drug development strategies
LA JOLLA—Just like your left and right hand exist as mirror images of each other, many biological molecules have their own form of left- and right-handedness, called chirality. Our DNA, for example, is made of right-handed chiral molecules which combine to form a right-handed double helix. The left-handed version would look like its mirror image, forming a helix that spins in the opposite direction.
The thing about nature, though—it tends to pick a side. On Earth, DNA and RNA exist only in their right-handed forms. Even when scientists construct synthetic left-handed versions of these molecules, the two groups behave as if on opposite sides of a mirror, unable to interact with each other.
But what if they could? What if a molecule could reach through the mirror and interact with the reflected world on the other side? What if this set off a chain reaction that got molecules on both sides working together in ways we’ve never seen before?

This is precisely what scientists at the Salk Institute have now achieved. In a study published on October 22, 2024, in Proceedings of the National Academy of Sciences, the researchers demonstrate the first cross-chiral exponential amplification of an RNA enzyme. Using sophisticated bioengineering techniques, they produced a chemical system in which left- and right-handed versions of an RNA enzyme can effectively “reach through the mirror” and replicate each other. Through this cross-chiral self-replication, the amount of both molecules increases exponentially and indefinitely—something rarely seen outside of biology.
In fact, NASA defines life as "a self-sustaining chemical system capable of Darwinian evolution." The researchers say this is the first evidence of a life-like chemical system that operates on both sides of the mirror of chirality.
“Exponential self-replication is necessary for growth and evolution in every living system,” says co-corresponding author and Salk President Gerald Joyce. “Cells don’t just make more of themselves; they make exponentially more of themselves, and that fast growth is what drives competition, natural selection, and evolution. We’ve now shown that we can engineer forms of exponential genetic self-replication that are not yet life but are on the path to it, and are built on interactions between left- and right-handed molecules.”
While cross-chiral self-replication is unlikely to occur spontaneously in nature, the discovery that it can be engineered in a laboratory setting suggests scientists could one day synthesize an artificial living system that uses both left- and right-handed molecules. This would create the opportunity to study an entirely new form of biochemical evolution, and could also lead to the development of cross-chiral therapeutics and biotechnologies.
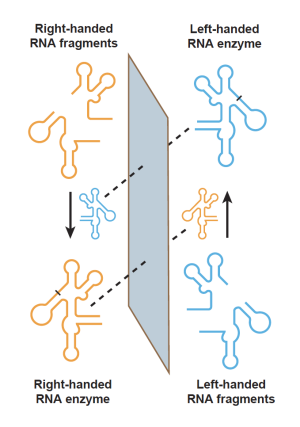
“We’ve come to expect that life on our planet and other planets will be single-handed, but our work suggests that doesn’t have to be true for a bioengineer,” says co-corresponding author David Horning, a senior staff scientist in Joyce’s lab. “We’re essentially exploring the boundaries of what biology can be, and based on this study, it seems that our definition of life doesn’t have to be as narrow in the lab as it is in nature.”
To achieve cross-chiral exponential amplification, co-first authors Wesley Cochrane and Grant Bare expanded on the lab’s pioneering methods for driving the directed evolution of RNAs. In this case, the system was used to produce an RNA enzyme that is very good at making the opposite-handed version of itself. Importantly, the same enzyme could now be further engineered to also make additional RNA products that carry out other valuable functions. The resulting cross-chiral autocatalytic system could have many applications in medicine and biomanufacturing.
For example, the authors say they could one day synthesize new left-handed RNAs that interact with right-handed molecules in the body in very specific, desired ways. Because these left-handed RNAs would go virtually undetected by the cell and immune system, they wouldn’t degrade as quickly as other drugs. They also wouldn’t be able to interact with any other right-handed molecules, reducing the likelihood of off-target side effects.
This cross-chiral strategy could inspire an entirely new class of therapeutics, diagnostics, and research tools. The Joyce lab has already developed systems to produce left-handed RNAs that bind to disease-related RNAs and proteins. Another project is exploring their use as signal amplifiers, allowing researchers and clinicians to detect trace amounts of specific molecules of interest, such as viral RNAs.
“It’s like having a parallel biology that exists next to our biology, but it’s designed entirely by us and nature can’t interfere with it,” says Horning. “Cross-chiral self-replication opens up a whole new world of biochemical possibilities, so we’re just beginning to imagine all of the ways we could use these mirrored molecules to our benefit.”
The work was supported by the National Science Foundation (MCB2114588) in collaboration with Professor Jon Sczepanski at Texas A&M University and the National Institutes of Health Ruth L. Kirschstein National Research Service Award (F32GM146435) to Wesley Cochrane.
DOI: https://doi.org/10.1073/pnas.2413668121
JOURNAL
Proceedings of the National Academy of Sciences
AUTHORS
Wesley G. Cochrane, Grant A. L. Bare, Gerald F. Joyce, and David P. Horning
Office of Communications
Tel: (858) 453-4100
press@salk.edu
Unlocking the secrets of life itself is the driving force behind the Salk Institute. Our team of world-class, award-winning scientists pushes the boundaries of knowledge in areas such as neuroscience, cancer research, aging, immunobiology, plant biology, computational biology and more. Founded by Jonas Salk, developer of the first safe and effective polio vaccine, the Institute is an independent, nonprofit research organization and architectural landmark: small by choice, intimate by nature, and fearless in the face of any challenge.