
October 31, 2019
International collaboration improves method for culturing primate embryos to learn more about human development
International collaboration improves method for culturing primate embryos to learn more about human development
LA JOLLA—Little is known about the molecular and cellular events that occur during early embryonic development in primate species. Now, an internationally renowned team of scientists in China and the United States has created a method to allow primate embryos to grow in the laboratory longer than ever before, enabling the researchers to obtain molecular details of key developmental processes for the first time. This research, while done in nonhuman primate cells, can have direct implications for early human development.
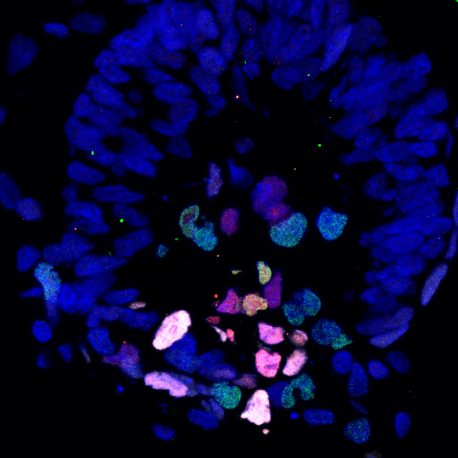
Click here for a high-resolution image.
Credit: Weizhi Ji/Kunming University of Science and Technology
The findings, published in Science on October 31, 2019, provide valuable insight into early embryonic development and potentially can help inform approaches to advance regenerative medicine in humans.
“Our study provides a first look into this black box of early development,” says Juan Carlos Izpisua Belmonte, co-corresponding author and a professor in Salk’s Gene Expression Laboratory. “We can now observe how cells progress through each embryonic stage and what factors they need to develop, which will aid in creating better options for the generation of a variety of cells and tissues.”
“To understand cellular and molecular mechanisms underlying primate gastrulation, we began monkey embryo culture experiments in China three years ago. Because of the team’s long-standing expertise on the systematic study of nonhuman primates and well-established reproductive research systems, such as an in vitro fertilization platform, we succeeded in achieving our goals. This can help to shed light on previously unknown aspects of human post-implantation development.” says Weizhi Ji, co-corresponding author, professor and dean of the Institute of Primate Translational Medicine of Kunming University of Science and Technology, in China.
The scientists wanted to study an early developmental milestone called gastrulation, which occurs when a developing embryo transforms into a multilayered structure, called the gastrula, from which all future tissues and organs will be derived. One layer will become the lungs, gastrointestinal tract and liver; another will become the heart, muscles and reproductive organs; and a third will become the skin and nervous system. Yet, scientists did not know the molecular and cellular drivers of this process in primates, largely due to limited access to early embryos.

Click here for a high-resolution image.
Credit: Salk Institute
“Our goal was to culture a primate embryo from an early timepoint in order to study the process of development,” says Jun Wu, a co-author of the paper and assistant professor at University of Texas Southwestern Medical Center. “We wanted to monitor the embryos every day to observe their shape, size and migration patterns as well as how they generate different types of cells during early primate development.”
To better study this critical transformation, the scientists modified a previously established embryo culture protocol to allow an early primate embryo to develop in laboratory conditions for up to twenty days; previously researchers had only been able to maintain cultured primate embryos prior to the second week of gestation. Using the new protocol, the team found that the cells within cultured embryos exhibited clear developmental trajectories towards each layer of the gastrula, and the results revealed some of the molecular details required for this growth. The data could also be used as a resource to aid in extending the cultured embryo duration past twenty days in order to better study stem- cell differentiation (specialization).
“These results illuminate some of the regulation networks and signaling pathways that are crucial to development in primates,” says Izpisua Belmonte. “This system provides a foundation and resource for developing better strategies to examine early primate development in both health and disease, in the laboratory.”
Other authors included Yuyu Niu, Nianqin Sun, Chang Li, Chenyang Si, Jinkuan Wei, Yu Kang, Wei Si, Hong Wang, E Zhang, Lu Zhao, Ziwei Li and Tao Tan of Kunming University of Science and Technology; and Ying Lei, Xi Dai, Chuanyu Liu, Zhihao Huang and Longqi Liu of BGI-Shenzhen.
The work was funded by the National Key Research and Development Program (2016YFA0101401), the National Natural Science Foundation of China (81760271), Major Science and Technology Projects of Yunnan Province (2017ZF028) and Key Projects of Basic Research Program in Yunnan Province (2017FA010). Work in the laboratory of Juan Carlos Izpisua Belmonte was supported by The Moxie Foundation.
DOI: 10.1126/science.aaw5754
JOURNAL
Science
AUTHORS
Yuyu Niu, Nianqin Sun, Chang Li, Ying Lei, Zhihao Huang, Jun Wu, Chenyang Si, Xi Dai, Chuanyu Liu, Jinkuan Wei, Longqi Liu, Yu kang, Wei Si, Hong Wang, E Zhang, Lu Zhao, Ziwei Li, Juan Carlos Izpisúa Belmonte, Weizhi Ji and Tao Tan
Office of Communications
Tel: (858) 453-4100
press@salk.edu
Unlocking the secrets of life itself is the driving force behind the Salk Institute. Our team of world-class, award-winning scientists pushes the boundaries of knowledge in areas such as neuroscience, cancer research, aging, immunobiology, plant biology, computational biology and more. Founded by Jonas Salk, developer of the first safe and effective polio vaccine, the Institute is an independent, nonprofit research organization and architectural landmark: small by choice, intimate by nature, and fearless in the face of any challenge.